Original Article
Burden of Hepatocellular Carcinoma and Pattern of Hepatitis B and C Presentation among HCC Patients: A Retrospective Study from a Tertiary Care Hospital in Karachi, Pakistan
Authors: Navaira Ali, Naila zahid, Taimur Ashraf Arain, Maheen Zahid
DOI: https://doi.org/10.37184/lnjcc.2789-0112.4.9
Year: 2022
Volume: 4
All articles are published under the Creative Commons Attribution License
Abstract
Background: Hepatocellular carcinoma (HCC) is the most commonly occurring cancer of the liver. HCC ranks fifth from the top among the most prevailing cancers in men globally. It stands second among the major causes of mortality associated with cancer in the world. The majority of the cases of hepatocellular carcinoma (HCC) are due to cirrhosis developed after infection with the chronic hepatitis B virus (HBV) or hepatitis C virus (HCV).
Objective: To assess the burden of hepatocellular carcinoma and to study the pattern of hepatitis B and C in HCC patients who visited oncology outpatient department (opd) services in a tertiary care hospital in Karachi.
Materials and Methods: This is a retrospective study. The total number of patients visiting the Oncology OutPatient Department from July 2019 to June 2021 was recorded. Patients who had HCC of these were included in the study. The age, viral markers, and TNM staging of the patients were recorded. Both male and female patients belonging to any age group were included in the study.
Results: During two years, a total of 2,102 patients visited oncology outpatient services for different cancers. Out of these, 50 patients had a diagnosis of HCC and were included in the study. The mean age of the patients was 59.76±10.16 years. 70% of the patients were male and 30% were female. 60% of the patients were positive for Hepatitis C, while 36% of the patients had all viral markers negative.
Conclusion: HCC is a fairly common malignancy but screening programs across the world are hampered by low rates of utilization. Infection with hepatitis B and C is frequently associated with the development of HCC. If proper and adequate screening measurements are taken in time for patients who are at greater risk for hepatocellular carcinoma, better survival rates could be seen in Pakistan.
Keywords: Hepatocellular carcinoma (HCC), hepatitis B virus (HBV), hepatitis C virus (HCV).
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most commonly occurring cancer of the liver. HCC ranks fifth from the top among the most prevailing cancers in men globally. Among the female population, it is the seventh most common cancer. Around half a million new cases of liver cancer are diagnosed annually worldwide. Hepatocellular carcinoma is the second major cause of death related to cancer in the world [1, 2]. In 2020, around 609,596 new cases of HCC were diagnosed in Asia, contributing to about 72.5% of the total occurrence of liver cancer in the world. Keeping in view the mortality, the number of deaths attributed to liver cancer in Asia was 566,269. This accounts for 72.4% of the total liver malignancy deaths in the world [3].
In the majority of the cases of HCC, an association has been found with cirrhosis resulting because of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. This postulates that chronic liver disease (CLD) resulting due to hepatitis B virus (HBV) or hepatitis C virus (HCV) is blameworthy for most Hepatocellular carcinoma cases. This further suggests that effective preventive measures can lower the incidence of HCC [4].
The geographic parts of the world where the risks of HCC are highest are South-East Asia and sub-Saharan Africa because in these regions here hepatitis B is greatly endemic [5]. Pakistan bears one of the world’s greatest burdens of chronic hepatitis. The country also records one of the highest death counts due to liver failure and HCC. Many studies have been conducted in Pakistan about the incidence of HBV and HCV among the population as well as the risk factors involved [6, 7].
The objective of this study is to assess the burden of hepatocellular carcinoma and to study the pattern of hepatitis B and C in HCC patients who visited oncology outpatient department services in a tertiary care hospital in Karachi.
MATERIALS AND METHODS
This is a retrospective study. From July 2019 to June 2021, a total of 2,102 patients visited Oncology OPD diagnosed with different types of cancers. Out of these, 50 patients had hepatocellular carcinoma. These patients were included in our study. The age and viral markers of the patients were recorded. TNM staging of the disease of patients was noted and the stage was calculated and recorded. Child-Pugh Classification of those patients was calculated whose bilirubin, albumin, and International Normalized Ratio of blood was available. The said classification of all patients was not available as some of them did not follow up later on. Patients of both genders were included in the study. Patients belonging to any age group were included in the study. Those patients who had more than one primary malignancy including HCC were not included in the study.
RESULTS
Socio-demographic aspects: During two years, a total of 2,102 patients visited oncology outpatient services for different cancers. Out of these, 50 patients had a diagnosis of HCC and were included in the study. The youngest patient was 33 years old and the oldest patient was 82 years old. The mean age of the patients was 59.76±10.16 years. 70% of the patients were male and 30% were female.
Clinical Features:
58% of the patients had advanced metastatic hepatocellular carcinoma. 6% belonged to stage 1, 18% belonged to Stage 2 and an equal percentage belonged to Stage III patients. Child-Pugh Classification of 30 patients was available. Out of these 18 patients were Class A and 12 patients were Class B. 60% of the patients were positive for Hepatitis C, while 36% of the patients had all viral markers negative.
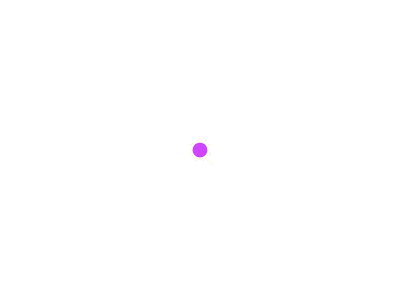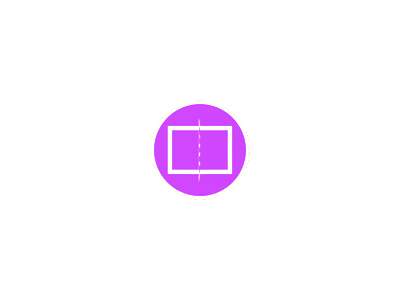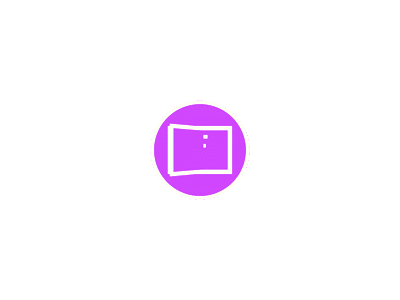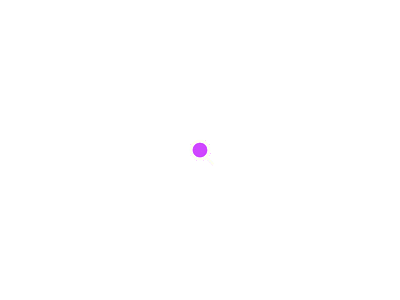
As shown in Fig. (1), 60% of the patients were positive for Hepatitis C, 2% were positive for Hepatitis B, 2% again were positive for both Hepatitis B and C while 36% of the patients had all viral markers negative.
Fig. (1): Shows percentages of positive and negative viral markers.
DISCUSSION
Liver Cancer happens to be the most swiftly increasing cancer in both genders in the United States. Since 1980, the rate of incidence has more than tripled. From 2006 to 2015, the rate has been increasing by about 3% per year. The American Cancer Society (ACS) has estimated that around 41,260 new cases of liver cancer will be diagnosed that include those of the intrahepatic bile duct too. Approximately three-fourths of these are estimated to be HCC [8]. ACS also estimates that 30.520 deaths will occur from liver cancer in 2022 [8]. Our study showed that out of 2102 new cancer patients visiting oncology OPD from June 2019 to July 2021, 50 were diagnosed with Hepatocellular carcinoma.
Speaking worldwide, HCC occurred more often in males than in females with a ratio of 2.4:1 [9]. In the study we conducted, 70% of the patients were male.
The average age at which HBV-associated HCC is diagnosed is 50 years [10]. Our current study showed that the mean age of the patients was 59.76 years. 78% of our patients had ages above 50 years while 22% were younger than 50 years.
Hepatitis C virus infection has lately been acknowledged to be a major risk factor for hepatocellular carcinoma. Evidence has been found by the detection of both antibodies to HCV (anti-HCV) and HCV RNA in the serum of a significant number of patients with HCC around the world. In a normal liver of a healthy person, any inflammatory process revitalizes the growth and repair of the cells. This restores the normal liver architecture. However, if the liver is exposed to this process of inflammation chronically, the balance between damage and regeneration in the liver is deranged. This can lead to excessive scar tissue formation which is called fibrosis. In chronic situations, the worsening of this fibrosis will eventually lead to cirrhosis which is identified by abnormal liver architecture. Cirrhosis often leads to HCC.
El Serag wrote in his report that chronic HBV infection has been found responsible for approximately 50% of the total incidence of HCC. He also reported that more or less all childhood HCC occurs after chronic HBV infection. HBV also happens to be a ruling risk factor in most regions of Asia and Sub-Saharan Africa that have a lofty incidence of HCC. Japan is an exception where the major hazard factor for HCC is chronic Hepatitis C infection instead. The seroprevalence of HB surface antigen (HBsAg) among persons with HCC has a wide variation. The seroprevalence is 3% in Sweden and 10% in the United States. It is 10%-15% in Japan and 70% in South Korea [11, 12].
The CDC Recommendation for Hepatitis C screening among adults in the United States suggests at least once in a lifetime Hepatitis C screening for all adults aged 15 years and older. The recommendation also suggests that all pregnant women during each pregnancy should be screened for Hepatitis C except in regions where the prevalence of HCV infection (HCV RNA positivity) is less than 0.1% [13]. Our data also showed that 60 percent of the patients with HCC were hepatitis C positive. 2% of our patients were hepatitis B positive and the same percentage had both viral markers positive.
Detection of hepatocellular carcinoma at an early stage is very crucial for a favorable clinical outcome.
That is the reason that multiple professional societies recommend screening patients who are at risk for HCC, like all patients with cirrhosis and subgroups of patients with chronic hepatitis infections [14-16]. For screening, the modality of choice is semiannual abdominal ultrasonography. Using alpha-fetoprotein with it helps in detecting HCC earlier.
The Japan Society of Hepatology revised the algorithm for HCC surveillance and diagnosis as shown in the following figure (Fig. 2) [17].
HCC usually remains latent and is diagnosed at a later stage worldwide16. Our data showed similar results with approximately 58% of patients belonging to Stage IV. An interesting finding of our study was that 72.4% of the male patients had stage IV disease of HCC. In females, the commonest stage was II attributing to 55.6% of female patients.
Fig. (2): showing an algorithm for surveillance and diagnosis in the fourth version of the Japan Society of Hepatology Clinical Practice Guidelines for Hepatocellular Carcinoma (4th JSH-HCC Guidelines) [11].
The incidence of HCC due to non-alcoholic steatohepatitis (NASH), has been rising [18]. Approximately 3-15% of cases of obese NASH lead to cirrhosis. About 4-27 percent of NASH cases with cirrhosis progress to HCC [19]. The yearly incidence of Hepatocellular Carcinoma among NAFLD patients is approximately 1.8 /per 1,000 person-years [20]. Those patients who have NASH possess a higher risk of developing HCC. These patients should, therefore, be registered in a screening program. Ultrasonography, CT, and MR spectroscopy are useful for this purpose [21]. There are a few challenges associated too. One of these with NASH-induced HCC is that half of the cases arise in non-cirrhotic patients. Such patients should be identified too to screen them for HCC.
The most accurate available data for the advantages of HCC screening is concluded from a large Randomized Control Trial that deduced that screening patients with chronic HBV ameliorated early tumor detection (stage I, 60.5% vs. 0%). It also improved the receiving of curative treatment (resection, 46.5% vs. 7.5%), and overall survival (37%; hazard ratio 0.63; 95% CI, 0.41-0.98) as compared to not screening [15].
STUDY LIMITATION
Complete detail of a few patients was not available for Child-Pugh classification; hence all patients were not assigned the classification. This is a limitation of our study.
CONCLUSION
HCC is a fairly common malignancy but screening programs across the world are hampered by shallow usage rates. Infection with hepatitis B and C is frequently associated with the development of HCC. Multiple studies have shown that the countries where intensive screening programs including ultrasonography and α-fetoprotein (AFP) are implemented, higher rates of survival have been observed in patients. Though studies are being conducted regarding the potential impairment related to screening too, the data evaluating physical or financial harms of HCC screening are restricted. If proper and adequate screening measurements are taken in time for patients who are at risk for hepatocellular carcinoma, better survival rates could be seen too in Pakistan. Here, it is important to point out that eliminating the cause of the situation is always better than treating the results later. Strategies should be devised that pave the way to block the transmission of HBV infection. Implementation of the hepatitis B vaccine also has helped in preventing perinatal and horizontal transmission of the disease. There is a clear need for a comprehensive long-term safety profile of antiviral prophylaxis.
ETHICAL APPROVAL
This type of study does not require an ethical approval.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA
The authors unanimously confirm that data supporting
the results of this study are available in the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
None.
AUTHOR’S CONTRIBUTION
All the authors contributed equally to the publication of this article.
REFERENCES
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer CA Cancer J Clin 2011; 61(2): 69-90.
DOI: https:// doi.org/10.3322/caac.20107
2. World Health Organization Mortality Database. WHO Statistical Information System. 2008. Available From: https://www.who.int/ data/data-collection-tools/who-mortality-database
3. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. 2020. Available online: https://gco.iarc.fr/today/ home
4. Bosch FX, Ribes J, Cleries R, Diaz Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005; 9(2): 191-211.
DOI: https://doi.org/10.1016/j.cld.2004.12.009
5. Hou J, Liu Z, Gu F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int J Med Sci 2005; 2(1): 50-7.
DOI: https://doi. org/10.7150%2Fijms.2.50
6. Mehmood S, Raza H, Abid F, Saeed N, Rehman HM, Javed S, et National prevalence rate of hepatitis B and C in Pakistan and its risk factors. J Public Health (Berl.) 2020; 28: 751-64.
DOI: https://doi.org/10.1007/s10389-019-01081-5
7. Abbas Z, Jeswani NL, Kakepoto GN, Islam M, Mehdi K, Jafri W. Prevalence and mode of spread of hepatitis B and C in rural Sindh, Trop Gastroenterol 2008: 29(4): 210-6
8. Cancer Facts & Figures 2022. American Cancer Society 2022. Available from: https://www.cancer.org/latest-news/facts-and- figures-2022.html
9. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN Int J Cancer 2010; 127(12): 2893-917.
DOI: https://doi. org/10.1002/ijc.25516
10. Bosch FX, Ribes J, Borras Epidemiology of primary liver cancer. Semin Liver Dis 1999; 19: 271-85.
DOI: https://doi. org/10.1055/s-2007-1007117
11. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular Gastroenterology 2012; 142(6): 1264-73.e1.
DOI: https://doi.org/10.1053/j.gastro.2011.12.061
12. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, CA Cancer J Clin 2005; 55(2): 74-108.
DOI: https://doi. org/10.3322/canjclin.55.2.74
13. Hepatitis C information, Centers for Disease Control and
14. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular Hepatology 2018; 67(1): 358-80.
DOI: https://doi. org/10.1002/hep.29086
15. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular J Hepatol 2018; 69(1): 182-236.
DOI: https://doi.org/10.1016/j. jhep.2018.03.019
16. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 Hepatol Int 2017; 11 (4): 317-70.
DOI: https://doi.org/10.1007/s12072-017-9799-9
17. Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015; 45(2).
DOI: https://doi. org/10.1111/hepr.12464
18. Oda K, Uto H, Mawatari S, Ido Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: A review of human studies. Clin J Gastroenterol 2015; 8(1): 1-9.
DOI: https://doi.org/10.1007/s12328-014-0548-5
19. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty Hepatology 2010; 51(5): 1820-32.
DOI: https://doi.org/10.1002/ hep.23594
20. Teng YX, Xie S, Guo PP, Deng ZJ, Zhang ZY, Gao W, et al. Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Current Progresses and Challenges. J Clin Transl Hepatol 2022; 10(5): 955-64.
DOI: https://doi.org/10.14218/jcth.2021.00586
21. Dhamija E, Paul SB, Kedia S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J Med Res 2019; 149(1): 9-17.
DOI: https://doi.org/10.4103/ ijmr_1456_17