Review Article
The Significance of Androgen Receptor in Oncogenesis and Cancer Treatment: Emphasis on Oral Squamous Cell Carcinoma
Authors: Yumna Adnan, Syed Muhammad Adnan Ali, Hasnain Ahmed Farooqui, Hammad A. Kayani
DOI: https://doi.org/10.37184/lnjcc.2789-0112.3.2
Year: 2021
Volume: 3
Received: Sep 20, 2021
Revised: Nov 25, 2021
Accepted: Dec 30, 2021
Corresponding Auhtor: Syed Muhammad Adnan Ali (syed.adnan@aku.edu)
Abstract
The androgen receptor (AR) is a transcription factor that is dependent upon ligand activation and is a member of the nuclear receptor superfamily. AR has been found to have a role in cancers of the prostate, breast, ovarian, nasal cavity, and lung. It has been seen that AR plays a significant role in both the early and later stages of prostate cancer as AR is expressed in almost every primary prostate cancer. An evolving prognostic and therapeutic role of AR also exists in breast cancer as increased expression of AR has been linked to improved survival. In the case of OSCC, the role of hormonal therapy as a prognostic marker remains to be explored. Some reports have failed to identify the expression of AR in oral cavity cancers or any significant associations, while others have predicted AR-expressing tumours to have a worse prognosis. This review narrates the current research on AR and how it can be applied in future research.
Keywords: Androgen receptor (AR), oncogenesis, cancer, oral squamous cell carcinoma.
INTRODUCTION
Androgen receptor (AR) is a member of the steroid nuclear receptor-ligand binding superfamily. It is a ligand-dependent nuclear transcription factor and is ubiquitously expressed throughout the entire body. AR is located at the sex chromosome Xq12 divided into 8 exons and over 90 kilobases long [1]. The main physiological role of androgen hormones and their respected target receptors is for the development of male secondary sexual characteristics and the male reproductive system. AR is expressed in a variety of tissues and performs multiple biological activities in bone, muscle, prostate, adipose tissue and the reproductive, cardiovascular, immune, neural and hematopoietic systems [2].
HISTORICAL BACKGROUND
A. A. Berthold was the scientist who first discovered the concept of androgen hormones and that they have a structure similar to that of other steroid hormones. In a classic experiment, he removed the testes of male chickens (castration) and observed a decrease in the development of secondary sexual characteristics [3]. Following that, Liao, Leininger (1965) [4] discovered that in prostate nuclei, the activity of RNA synthesis rapidly increases due to AR. Liao and Fang (1970) [5] showed for the first time the proteins to which androgen hormones and their types bind in order to carry out their function and named them as androgen receptors. Androgen hormones and receptors play a vital role in different stages of male sexual development and maintain their entire phenotype and genotype as well.
Androgen receptors are phosphoprotein in nature and after phosphorylation of the receptors, they allow attachment of the ligand-binding hormones and initiate transcription binding activity of the target genes [6].
MOLECULAR STRUCTURE OF AR
The human AR is an intracellular receptor ligand-binding protein. After molecular cloning, the primary structure of AR was revealed. The cDNA, made up of 2721 nucleotides, encodes a protein containing 917 amino acid residues and having a molecular mass of 98,845D. The coding region of AR is divided into 8 exons, and it has a similar organization to other steroid hormones such as estrogen receptors and progesterone receptors. The overall length of the gene is more than 90 kilobases [7]. The sequence which encodes the N-terminal region is located at one large exon. Two different small exons encode separately the two DNA-binding fingers. Five different exons contain information about the hormone- binding domain. The location of introns is similar to those reported for the chicken progesterone receptor and human epidermal growth factor-2 genes [8].
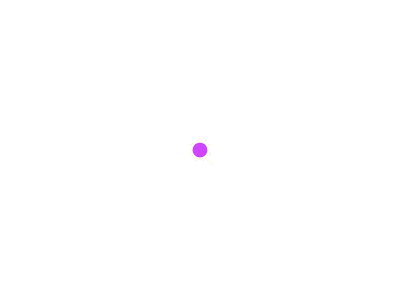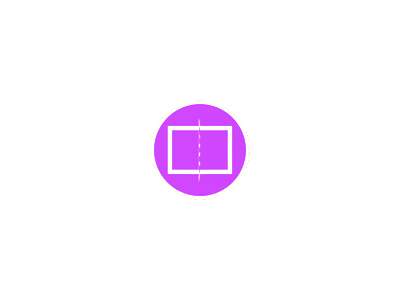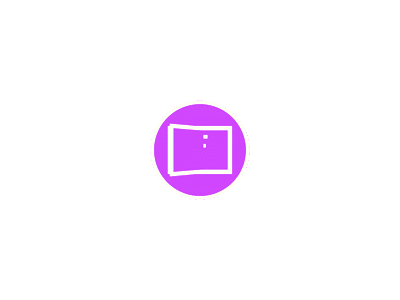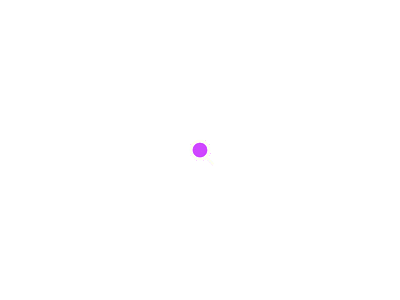
The structure of AR is elucidated in Fig. (1). Androgen nuclear receptors contain two structural subunits; a C-terminal ligand-binding domain (LBD) which is moderately conserved, and a DNA- binding domain (DBD), which is a much-conserved region. Following are the functions of both the ligand domains: The ligand-binding protein (LBD) performs multiple tasks: firstly, as the name suggests it has an interior specific
binding pocket for the target hormones or ligand. With
*Corresponding author: S. M. Adnan Ali, Department of Surgery, Aga Khan University Hospital, Karachi, Pakistan: syed.adnan@aku.edu
Received: September 20, 2021; Revised: November 25, 2021; December 30, 2021 DOI: https://doi.org/10.37184/lnjcc.2789-0112.3.2
this pocket, they regulate the hormones and ligands. Secondly, the LBD has a ligand, known as transcriptional Activation Function (AF2). AF2 can examine the
proteins, such as all the co-activators of the steroid hormones, so the co-activators can easily interact with the chromatin–remodelling protein and start the transcriptional mechanism. The fourth and last function of LBD is that it has a special potential of self-building such as dimerization (combining to 2 similar molecules) and tetramerization (combining of 4 similar molecules), which is important for increasing the strength of attraction and binding affinity of the ligand and the receptor on DNA elements which will, in turn, regulate transcription [9].
|
|
Fig. (1): Structure of androgen receptor [Sourced from Fujita and Nonomura (2019) [10]].
|
|
Fig. (2): Human Androgen Receptor ligand binding in complex with the ligand Metribolone (shown in blue) (R1881). RCSB PDB ID: 1E3G Using the Mol* viewer [11].
The second domain, DNA binding domain (DBD) consists of two non-equal zinc finger structures. These zinc atoms are attached with four cysteine residues and are essential for the folding and DNA binding stability and activities. Fig. (2) from the Protein Data Bank visualizes
the molecular structure of AR and the attachment of a ligand with an intrinsic region of AR. It is also important for the structural stability and functions of the domain. With very short sequences of amino acid, the LBD and DBD are connected, and that sequence is known as the hinge region. The property and function of the hinge region are still not well understood, however, it may take part in phosphorylation, which is necessary for the up- regulation of general transcriptional processes [9, 12].
Moreover, other sequences of amino acids are also present between the N-terminal and the DNA- binding domain. These sequences are known as the transcription activation function-1 (AF1). As compared to the moderately conserved domain, AF2, it appears to be a weakly conserved region in all the nuclear receptor superfamily. Its function and properties are also not well understood yet, but functionally it combines to work with AF2 to produce a greater effect. AF1 also acts as a ligand-independent transcriptional activator [12].
BIOLOGICAL IMPORTANCE OF AR
Androgen hormones act on their specific androgen receptors which lead to gene expression. Just like other nuclear receptors, AR also regulates the transcription of specific genes by connecting with hormones control elements. These elements are present on the gene at the 5’ region, but in some conditions, especially in introns, they are present at the 3’ region. Most of the regulated genes contain Androgen Response Elements (AREs), which are located at the DNA-binding domain [13].
Androgen hormone binding with AR prompts the conformational changes within the ligand-binding domain, and along with that more phosphorylation occurs in the receptor. Afterward, the ligand AR connects with targets the androgen response elements at the regulatory domain of androgen-specific genes and then regulation of gene expression occurs [6]. Some co-activators are specific for receptors, and it may be essential for the expression of genes and some of them are used to stop the molecular expression of the receptor towards the specific co-activators. Along with the transcriptional activity of other nuclear receptors, AR activity may be induced by some of the specific co- activators, such as F-SRC-1, CBP/ p300, TIF2/GRIP1, RAP6/BAG-1, ARA70 and RIP-14s0. Some of the co- activators are interconnected with AR directly or they may be connected with other co-activators and then bind with AR [13]. Fig. (3) illustrates the AR signalling pathway inside a cell.
AR has two ligands, testosterones, and their stronger version 5-dihydrotestosterone. The latter, 5-dihydrotestosterone, is a metabolite of testosterone and is thought to be 3 to 6 times more powerful than testosterone because it has a strong binding affinity to the receptor [15]. Both the hormones activate the target gene expression and transcribe it by the process of binding
Fig. (3): Schematic of AR signaling pathways. Abbreviations used in full: PI3K: phosphoinositide 3-kinase, GPCRs: G protein-coupled receptors, SHBGR: sex hormone-binding globulin receptor, cAMP: cyclic adenosine monophosphate, PKC: protein kinase C. Modified from Pietri, Conteduca (2016) [14].
with AR. There are different types of co-regulators and co-regulator composites that help and control the ligand- dependent transcriptional process. The composites are classified into three characteristics according to their performance: first, the multiplex system of co-activators which has a physical connection with the transcription factors and polymerase II. This interaction controls and regulates the transcription. Second, the co-activator multiplex system has a modified histone tail covalently bonded. Third, is the process of ATP-dependent dynamic remodeling structure of chromatin proteins [16].
Multiple effects of androgen hormones are generated by binding of AR with cis-acting androgen response elements in the affected target gene. With time, it became clear that in many genes which do not have androgen response elements androgen may involve indirectly in gene expression. Different forms of indirect effects may take place, including, a modification that occurs in the expression of secondary transcriptional factors. Growth factors production may act as autocrine and paracrine responses [16]. Fig. (4) summarizes the mechanisms of ligand-dependent AR functions.
Testosterone and dihydrotestosterone (DHT) are the two main types of androgen hormones. Androgen hormones are made in two organs of the human body: the testis and adrenal glands. The Leydig cells of the testis synthesize testosterone and the adrenal glands synthesize
Fig. (4): Mechanisms of ligand-dependent androgen receptor (AR) action: (1) DNA binding-dependent (DBD) and (2) non-DNA binding (DBD)-dependent. (AP-1 – activator protein 1) (Rana et al., 2014).
androstenedione and dehydroepiandrosterone (DHEA). The majority of testosterone binds to sex hormone- binding globulin in the serum and the leftover 1%-2% remains as free testosterone [10].
Testosterone further modifies into its active form such as DHT, by 5α-reductase enzyme, and then to estradiol with the help of aromatase and then utilized in biological activities. Testosterone and dihydrotestosterone play their part with the help of androgen receptors which are ligand-dependent nuclear transcription factors. ARs are present on the X-chromosome, and they are expressed in a variety of tissues and perform multiple biological activities in bone, muscle, prostate, adipose tissue, and the reproductive, cardiovascular, immune, neural and hematopoietic systems. DHT has more biological activities as compared with testosterone [2].
If androgen hormone deficiency occurs in males, it leads to many severe consequences, such as a decrease or ultimate cease of testosterone levels. Androgen deficiency is generally classified into classical hypogonadism (organic androgen deficiency) and age- associated decrease in testosterone. Testosterone usually decreases with age and that is one of the reasons older men suffer from multiple syndromes such as diabetes, prostate cancer, heart disease, mood changes and many other diseases. It is suggested for older men to control their comorbidities such as the use of antiglycaemic agents to control diabetes and these acts can have multiple health benefits including a return of testosterone to normal levels [2].
AR also plays a significant role in the development of several parts of the central nervous system. It also contributes in the formation and function of the circuit of CNS in a hypercritical condition, and protects the brain from noxious agents, maintains the regulation and development of function in adulthood. It also helps in the development and maintenance of reproductive and non- reproductive behaviour such as sexuality, aggression and different emotions and along with estrogen, it deals with gender-related differences [16].
ROLE OF AR IN CANCER
AR has been found to have a role in cancers of the prostate, breast, ovarian, nasal cavity, and lung. Table 1 lists studies in various cancers regarding the expression of AR. Mostly, AR is linked to prostate cancer, ever since the discovery that prostate cancer responded to androgen withdrawal. Prostate cancer is the most common cancer of solid organs in males and contributes to a heavy burden of cancer-related deaths each year [2]. The prostate cancer incidence rate has been seen as very high in the United States and other western countries [17]. It has been seen that AR plays a significant role in both the pre- and later stages of prostate cancer development. AR expression is present in almost every primary prostate cancer. In vivo and in vitro studies on humans and animals suggested that
increased expression of AR in patients can lead to the development of prostate cancer [18-20]. Another study also revealed that there was an association between prostate cancer recurrence and depletion of androgen receptors [21, 22]. Androgen deprivation therapy is usually able to suppress the majority of prostate cancers, although some are seen to progress to castration-resistant prostate cancer, which has the ability to continue growing under reduced androgen levels. In castration-resistant prostate cancers, the AR gene is dysregulated in about 63% of tumours using a variety of mechanisms. The development of castration- resistant prostate cancer arises due to point mutations in the AR gene (15%-30% of cases), amplification of the AR gene (30% - 50% of cases), changes in the androgen biosynthesis processes, changes in the AR cofactor and androgen receptor variants [10].
An evolving prognostic and therapeutic role of AR exists in breast cancer. About 60% to 80% of all breast cancers express AR and among these, there is a higher prevalence for tumours that also express estrogen
Table 1: Role of androgen receptor in various cancers.
receptors. Increased expression of AR has been linked to improved survival in breast cancer patients that express both androgen and estrogen receptors [23]. Furthermore, breast cancers are defined based on molecular profiling as well and the expression of AR differs among the molecular subtypes. One of these subsets of breast cancers, known as basal-like cancers is known to have about one-third AR positivity and may benefit from androgen-receptor targeted therapy [24].
Although circulating androgens are found in both pre- and post-menopausal women, an increased risk for the development of breast cancer was detected in pre- menopausal women who had high levels of circulating testosterone [25]. Moreover, in post-menopausal women, a higher than baseline testosterone level in the serum was found to be an independent prognostic factor for distant metastases and local relapse [26]. But not all studies have agreed that increased serum levels of androgens increase the risk for breast cancer. A study by Adly and colleagues has argued that higher levels of estrogen and not androgen leads to an increased risk of
|
S. No |
Author (Year) |
Samples |
Technique |
Findings |
|
1 |
Hwang, Mills (1998) [30] |
24 nasopharyngeal angiofibromas |
ICC |
Expression of AR was found in 18 (75%) out of 24 cases. Positive staining was seen in stromal and endothelial nuclei. This was the first evidence of the role of AR in angiofibromas. |
|
2 |
Montag, Tretiakova (2006) [31] |
13 nasopharyngeal angiofibromas |
IHC |
Out of 13, only 5 cases (39%) were positive of AR. Staining was observed weakly in stromal cells only, with negatively stained endothelial and pericytic cells. |
|
3 |
Lee, Rosen (2005) [32] |
TMA of 322 primary ovarian carcinomas |
IHC |
AR expression was found in 43.7% of all ovarious carcinomas and was highest in serous carcinomas (47.5%). AR expression was not linked to better survival of patients. |
|
4 |
Chodak, Kranc (1992) [19] |
106 sections from 57 patients; 3 normal prostate epithelium, 23 benign prostatic hyperplasia and 31 untreated prostate cancer |
IHC |
AR expression was found mainly in epithelial prostatic cells. AR expression was significantly higher in well-differentiated adenocarcinoma as compared to moderate and poorly differentiated. |
|
5 |
De Winter, Trapman (1990) [33] |
26 primary prostatic carcinomas |
IHC |
AR homogenous expression was found in 18 (70%) and heterogeneous expression was found in 7 (27%). The numbers of stained cells were decreased in more aggressive tumours (Grade III). |
|
6 |
Qiu, Leuschner (2008) [34] |
232 prostatic adenocarcinoma without neoadjuvant hormonal therapy or chemotherapy |
IHC |
Mean percentage of AR+ cells in cancer tissue was about 90% compared to normal prostate tissue which was 85%. AR expression was not correlated with any clinical parameters. |
|
7 |
Ogawa, Hai (2008) [35] |
227 primary breast cancers |
IHC |
Positivity rate for AR expression was 62.6% and it was significantly higher in small tumours, tumours that were negative for lymph node metastasis, histologically low-grade tumours and invasive ductal carcinomas. |
|
8 |
Park, Koo (2010) [36] |
413 primary breast cancers |
IHC |
AR expression was seen in 72.9% of breast cancers and was significantly associated with smaller tumours and those of a lower histologic type. |
|
9 |
Meijnen, Peterse (2008) [37] |
238 ductal carcinoma in situ |
IHC |
AR expression was found in 60/163 (37%) of cases and was significantly associated with histological differentiation. |
|
10 |
Leitao, Soslow (2004) [38] |
25 patients of leiomyosarcomas, 19 benign uterine leiomyomatas |
IHC |
AR expression in benign uterine leiomyomata and leiomyosarcoma was 32% and 40% respectively. AR was found to be related to a lower risk of recurrence but was not associated with overall survival. |
|
11 |
Kaiser, Hofmann (1996) [39] |
52 primary lung cancer, and 29 lung cancer cell lines |
IHC, PCR |
AR positivity was detected in 9 cases (17%) of lung cancer and 12/17 (71%) cases in cell lines. |
ICC: Immunocytochemistry IHC: Immunohistochemistry TMA: Tissue microarray
PCR: Polymerase chain reaction
Table 2: Role of androgen receptor in oral cancer.
|
S. No |
Author & Year |
Samples |
Technique |
Findings |
|
1 |
Ojanotko-Harri, Forssell (1992) [40] |
11 normal oral mucosal tissues |
IHC |
AR was detected in all 11 oral mucosal biopsies (100%). |
|
2 |
Nasser, Faquin (2003) [41] |
78 salivary gland tumours |
IHC |
AR expression was present in 14/14 carcinoma ex pleomorphic adenomas, 6/6 salivary duct carcinomas, and 2/2 basal cell adenocarcinomas but in only 2/10 acinic cell carcinomas, mucoepidermoid carcinomas, and adenoid cystic carcinomas each = total expression 54%. AR expression was 0% in benign tumours. |
|
3 |
Mohamed, Aro (2018) [43] |
199 oropharyngeal squamous cell carcinomas |
IHC |
In this study, immune positivity of AR was seen in 16% (31/199), out of which 39% (12/31) were mild and 61% (19/31) had strong expression. AR expression was observed to be at the invasive front of tumour. |
|
4 |
Choi, Song (2019) [44] |
189 squamous cell carcinomas of the tongue |
IHC |
Out of 189, only 2 specimens showed AR-positive expression. |
|
5 |
Nehse and Tunn (1994) [47] |
18 normal oral mucosa & squamous cell carcinomas. |
radioligand assay |
AR was undetectable in 5 tumours specimen. As compared to normal mucosa, AR levels were higher in 3 tumours and lower/ undetected in 15 tumours. |
|
6 |
Ito, Ito (2009) [48] |
41 salivary gland tumours |
IHC |
Out of 41 cases, AR was expressed in 2 cases of pleomorphic adenoma, negative for all Warthin’s tumours, 2/30 positive for mucoepidermoid carcinoma and 2/30 positive for adenoid cystic carcinomas. |
|
7 |
Aquino, Collina (2018) [49] |
69 benign and malignant salivary gland tumours |
IHC |
This study demonstrated that nuclear expression of AR was present in 15 out of 61 (24%) cases of malignant and benign salivary gland lesions. |
|
8 |
Moriki, Ueta (2001) [50] |
4 SDC, 51 benign and other malignant salivary gland tumours. |
IHC |
100% of patients with SDC were positive for AR while other salivary gland tumours were negative for AR, except for 2 cases of carcinoma in pleomorphic adenoma. |
|
9 |
Fan, Wang (2000) [51] |
13 salivary duct carcinomas |
IHC, PAP |
In males, 100% cells of all samples were positive for AR expression, while in females 2/5 were 100% positive and another had 40% positive cells, remaining 1 case was negative. |
|
10 |
Fan, Melhem (2001) [52] |
12 salivary duct carcinomas |
IHC |
11/12 samples had a positive expression of AR (92%). AR may act via the epidermal growth factor receptor pathway to promote tumorigenesis in SDC. |
|
11 |
Masubuchi, Tada (2015) [42] |
32 salivary duct carcinomas |
IHC |
AR was expressed in 23 (71.9 %) of all cases. This was significantly associated with disease-free survival, but not overall survival, on the univariate analysis but AR did not hold true as an independent marker on the multivariate analysis. Patients with AR negative tumours had a worse prognosis than AR-positive patients. |
|
12 |
Marocchio, Giudice (2013) [45] |
60 OSCC, 2 OSCC cell lines |
IHC, WB and IF |
The immunopositive expression of AR was 26.6% (16) and was significantly linked to gender as 75% of all AR-positive patients were male. |
|
13 |
Wu, Luo (2015) [46] |
11 benign, 22 premalignant, and 21 malignant lesions of the oral cavity, 4 OSCC cell lines |
Cell culture, WB, TMA-IHC, RT- PCR. |
Low AR expression detected from IHC was 27% (3/11) in benign, 59% (13/22) in premalignant and 29% (6/21) in malignant lesions. High AR expression was detected in 0 benign, 9% (2/22) premalignant and 38% (8/21) malignant lesions. The difference of expression among tissue types was also statistically significant. Stimulation of AR caused an increase in tumorigenesis and cell growth while knockdown of AR resulted in an increase in apoptosis and a decrease in cell proliferation. |
|
14 |
Sygut, Bien (2008) [53] |
37 salivary gland tumours |
IHC |
AR showed nuclear positivity in 3/4 salivary duct carcinomas, 2/7 adenocarcinomas and 1/2 carcinoma ex pleomorphic adenoma. All AR-positive cases were male. No AR expression was seen in adenoid cystic carcinomas, acinic cell carcinomas and mucoepidermoid carcinomas. |
|
15 |
Nakajima, Kishimoto (2009) [54] |
10 Carcinoma ex pleomorphic adenoma and 23 pleomorphic adenomas |
FISH, PCR- RFLP |
Expression of AR was found in 9 out of 10 carcinoma ex pleomorphic adenoma samples and in 30.4% of pleomorphic adenoma samples. No amplification was detected in any samples. |
IHC: Immunohistochemistry IF: Immunofluorescence WB: Western Blotting TMA: Tissue microarray
RT-PCR: Reverse-Transcriptase polymerase chain reaction
SDC: Salivary duct carcinoma
FISH: Fluorescent In situ hybridization
PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism
breast cancer [27]. This variability in results could be due to the different analytical techniques used to measure the levels of androgens and in the interpretation of data. Given the existing information, it cannot be concluded without doubt that androgens are a risk factor for breast cancer by themselves, but it can be affirmed that they do play a role in estrogen-positive breast cancer, if not all breast cancers [28, 29].
Furthermore, there is evidence of the role of AR in nasopharyngeal angiofibroma as well. Nasopharyngeal angiofibroma is a very rare benign tumour, prevalent among adolescent boys and a large percentage of these tumours (75%) have shown overexpression of AR [30] though another study has found a much lower positivity percentage, at 39% [31].
Regarding ovarian carcinomas and their subtypes, it was concluded by Lee et al. [32] that although increased expression of AR was found in 43.7% of all 322 primary ovarian carcinomas, this expression was not linked to better chances of survival.
ROLE OF AR IN ORAL CANCER
Steroid hormones make a liable factor in various malignant neoplasms as many biological responses are governed by these hormones. The receptors of these hormones belong to the ligand-binding nuclear receptor superfamily, and they play a vital role in the development and progression of tumours. Many researchers studying oral squamous cell carcinoma (OSCC) and salivary gland tumours have come up with the conclusion that the expression of androgen receptor, belonging to the ligand-dependent nuclear hormone family, may have a part in the development of OSCC and other tumours of the oral cavity ( Table 2).
In a 1992 study based on oral mucosal biopsies from healthy individuals, AR was found to be expressed in all specimens [40]. AR was also expressed in 54% of malignant salivary gland tumours and 0% of benign salivary gland tumours, indicating that there may be a role for AR in the pathogenesis [41]. AR was also found to be significantly associated with disease-free survival on the univariate analysis by Masubuchi, Tada (2015) [42], but was not independently significant as proved on the multivariate analysis. Although AR expression was not associated with overall survival of 2 years, the authors did note that patients with AR negative tumours had a worse disease-free survival rate than those who had AR-positive tumours (p = 0.011).
Similarly for other cancers, Mohamed and colleagues tested 199 oropharyngeal squamous cell carcinomas for the presence of AR. It was found that AR was expressed in 16% (31/199) of tumours and 61% (19/31) of these AR-positive tumours has strong AR expression [43]. Additionally, it was revealed that AR was co-expressed with p16 but not with HPV: tumours that were p16 positive and HPV negative expressed AR, but tumours
that were p16 negative and HPV positive did not express AR. AR expression was also stronger in patients with current or past smoking habits [43].
Contrarily, some reports have failed to identify the expression of AR in oral cavity cancers or any significant associations. Choi et al. [44] studied 189 squamous cell carcinomas of the tongue and found AR-positive expression in only 2 specimens, concluding that hormonal receptors might not be playing a major role in oral cavity cancer. In another study by Marocchio and associates [45] on OSCC, it was found that significantly higher percentages of males had AR-positive OSCC as compared to females (p = 0.023).
Furthermore, Wu et al. [46] examined 4 OSCC cell lines and premalignant, malignant and benign lesions of the oral cavity and concluded that functional AR was present in OSCC and that its function was critical for promoting cell growth and tumorigenesis. This was confirmed when both mRNA and protein levels of AR were detected in all OSCC cell lines and a majority of clinical specimens. The expression of AR was also significantly higher in malignant samples as compared to benign or premalignant samples and this was statistically important (p = 0.0073). Stimulation of the AR by dihydrotestosterone caused increased cell growth by increased expression of Cyclin D1. Additionally, knockdown of AR expression in OSCC cell lines caused a reduction in proliferation and increase in apoptosis while tumorigenesis was inhibited. Surprisingly, cytoplasmic expression was noted in almost all premalignant and malignant cases [46].
ROLE OF AR IN CANCER TREATMENT
AR is being evaluated and used as a target for treatment in cancers of the breast and prostate. Bicalutamide and flutamide are anti-androgens that bind to the ligand- binding domain of AR and thus prevent the binding of other androgens to the domain. Other antiandrogens include nilutamide and steroidal cyproterone acetate, although bicalutamide is the most extensively studied [55]. In patients that have castration-resistant prostate cancer, a second-generation nonsteroidal antiandrogen called enzalutamide is used which has a greater binding affinity for the ligand-binding domain. A recent trial has confirmed that the survival of such patients is prolonged by about 4.8 months by using enzalutamide [56]. Other androgen-targeted agents are also being tested in clinical trials to reduce the expression of AR, for example, galeterone which causes a decrease in AR expression by degrading two variants of AR [10]. However, the addition of an antiandrogen to castration therapy produces only marginally better results as compared to castration alone [57].
Although not fully believed to be involved in the development of breast cancer AR is undeniably involved in breast cancer progression. AR is seen to induce growth in tumours that are negative for
estrogen receptors and also inhibits the proliferation of cells in tumours that are estrogen receptor-positive. In the subset of breast cancers in which AR activation prohibits tumour growth, synthetic and natural steroidal androgens have been used to act as ligands and activate AR. But steroidal androgens have side effects and AR modulators, for example, Enobosarm is now in clinical trials giving favorable results and much less of side effects. For breast cancer that is tamoxifen resistant or in triple-negative breast cancers, AR antagonists are the most used therapies, for example, bicalutamide and enzalutamide, which are first and second-generation respectively [28].
In the case of OSCC, the positive expression of AR detected by immunohistochemistry has been reported, but a role for hormonal therapy remains to be explored [45]. It was found by Dos Santos, Sibov (2004) [58] in a study focusing on males with OSCC, that certain polymorphisms of the first eon of the AR gene could be linked to an increased risk of OSCC. Furthermore, Wu, Luo (2015) [46] using OSCC cell lines, showed that AR promoted tumorigenesis and cell growth when stimulated by dihydrotestosterone, and if AR was reduced it caused an increase in apoptosis and a decrease in cell proliferation. The benefits of androgen deprivation therapy have been demonstrated in a patient with AR-positive relapse salivary gland carcinoma in which complete remission was achieved. This further underscores the potential of anti-AR therapy in cancers other than prostate and breast [59].
CONCLUSION
AR has a vital role in the normal functioning of the human body, as well as in the development of cancer by encouraging cell invasion and migration. Several highly prevalent cancers such as breast cancer, are being studied to identify and target AR and improve outcomes. Although immunohistochemistry is the most cost-effective and easily available technique of determining AR expression in tumours, the optimal cut- off points for AR positivity have not yet been defined and this remains an area of concern as studies continue to produce discordant results using various arbitrary criteria. Increased expression of AR, as detected by immunohistochemistry, has been considered a good prognostic marker for squamous cell carcinomas of the head and neck. However, adverse have also been observed for cancers of the salivary gland and oral cavity squamous cell carcinomas. Moreover, androgen- deprivation therapy involves reducing the level of androgens in the body through targeted hormonal therapies and has been seen to improve outcomes for symptomatic metastatic prostate cancer patients. In addition to protein expression, AR gene expression and phosphorylation status may be used complimentarily to select patients for targeted therapy. It is hereby cautiously recommended that oral and other cancer patients who express increased levels of AR may be good candidates
for androgen-deprivation therapy, and this should be considered as part of their clinical treatment protocol.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
None.
REFERENCES
1. NCBI. AR androgen receptor [ Homo sapiens (human) ]: U. S. National Library of Science; 2020 [Available from: https://www. ncbi.nlm.nih.gov/gene/367.
2. Davey RA, Grossmann M. Androgen receptor structure, function
and biology: from bench to bedside. Clin Biochem Rev 2016; 37:3.
3. Berthold AA. Transplantation der hoden. Arch Anat Physiol 1849:42-6.
4. Liao S, Leininger K, Sagher D, Barton R. Rapid effect of testosterone on ribonucleic acid polymerase activity of rat ventral prostate. Endocrinology 1965; 77:763-5.
5. Liao S, Fang S. Receptor-proteins for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitam Horm. 271970. p. 17-90.
6. Brinkmann A, Blok L, De Ruiter P, Doesburg P, Steketee K, Berrevoets C, et al. Mechanisms of androgen receptor activation and function. J Steroid Biochem Mol Biol 1999; 69:307-13.
7. Brinkmann A, Faber P, Van Rooij H, Kuiper G, Ris C, Klaassen P, et al. The human androgen receptor: domain structure, genomic organization and regulation of expression. J Steroid Biochem 1989; 34:307-10.
8. Kuiper G, Faber P, Van Rooij H, Van der Korput J, Ris-Stalpers C, Klaassen P, et al. Structural organization of the human androgen receptor gene. J Mol Endocrinol 1989; 2:R1-R4.
9. Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol 2007; 21:2855-63.
10. Fujita K, Nonomura N. Role of androgen receptor in prostate
cancer: a review. World J Mens Health 2019; 37:288-95.
11. Sehnal D, Bittrich S, Deshpande M, Svobodová R, Berka K, Bazgier V, et al. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res 2021.
12. Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol 2007; 69:201-20.
13. Hiipakka RA, Liao S. Molecular mechanism of androgen action.
Trends Endocrinol Metab 1998; 9:317-24.
14. Pietri E, Conteduca V, Andreis D, Massa I, Melegari E, Sarti S, et al. Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr Relat Cancer 2016; 23:R485-98.
15. Hamdi M, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2‐dependent mechanism. J Physiol 2011; 589:3623-40.
16. Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas 2009; 63:142-8.
17. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA
Cancer J Clin 2010; 60:277-300.
18. De Winter JR, Janssen P, Sleddens H, Verleun-Mooijman M, Trapman J, Brinkmann AO, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 1994; 144:735.
19. Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang
C. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol 1992; 147:798-803.
20. Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer 1991; 67:3057-64.
21. Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res 2001; 61:423-7.
22. Kim D, Gregory CW, French FS, Smith GJ, Mohler JL. Androgen receptor expression and cellular proliferation during transition from androgen-dependent to recurrent growth after castration in the CWR22 prostate cancer xenograft. Am J Pathol 2002; 160:219-26.
23. Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor- positive breast cancer: results from the Breast International Group Trial 1–98. Breast Cancer Res 2019; 21:30.
24. Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol 2011; 24:924-31.
25. McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer 2014; 21:T161-T81.
26. Berrino F, Pasanisi P, Bellati C, Venturelli E, Krogh V, Mastroianni A, et al. Serum testosterone levels and breast cancer recurrence. Int J Cancer 2005; 113:499-502.
27. Adly L, Hill D, Sherman ME, Sturgeon SR, Fears T, Mies C, et al. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer 2006; 119:2402-7.
28. Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol (Lausanne) 2018; 9:492.
29. Chen M, Yang Y, Xu K, Li L, Huang J, Qiu F. Androgen receptor in breast cancer: From bench to bedside. Front Endocrinol (Lausanne) 2020:573.
30. Hwang HC, Mills SE, Patterson K, Gown AM. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Mod Pathol 1998; 11:1122-6.
31. Montag AG, Tretiakova M, Richardson M. Steroid hormone receptor expression in nasopharyngeal angiofibromas: Consistent expression of estrogen receptor β. Am J Clin Pathol 2006; 125:832- 7.
32. Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol Oncol 2005; 96:671-7.
33. De Winter JAR, Trapman J, Brinkmann AO, Boersma WJ, Mulder E, Schroeder FH, et al. Androgen receptor heterogeneity in human prostatic carcinomas visualized by immunohistochemistry. J Pathol 1990; 160:329-32.
34. Qiu YQ, Leuschner I, Braun PM. Androgen receptor expression in clinically localized prostate cancer: immunohistochemistry study and literature review. Asian J Androl 2008; 10:855-63.
35. Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol 2008; 13:431-5.
36. Park S, Koo J, Park H, Kim J-H, Choi S-Y, Lee J, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol 2010; 21:488-92.
37. Meijnen P, Peterse J, Antonini N, Rutgers ET, Van De Vijver M. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer 2008; 98:137-42.
38. Leitao MM, Soslow RA, Nonaka D, Olshen AB, Aghajanian C, Sabbatini P, et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer 2004; 101:1455-62.
39. Kaiser U, Hofmann J, Schilli M, Wegmann B, Klotz U, Wedel S, et al. Steroid-hormone receptors in cell lines and tumor biopsies of human lung cancer. Int J Cancer 1996; 67:357-64.
40. Ojanotko-Harri A, Forssell H, Laine M, Hurttia H, Bläuer M, Tuohimaa P. Immunohistochemical detection of androgen receptors in human oral mucosa. Arch Oral Biol 1992; 37:511-4.
41. Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors: frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am J Clin Pathol 2003; 119:801-6.
42. Masubuchi T, Tada Y, Maruya S-i, Osamura Y, Kamata S-e, Miura K, et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol 2015; 20:35-44.
43. Mohamed H, Aro K, Jouhi L, Mäkitie A, Remes S, Haglund C, et al. Expression of hormone receptors in oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2018; 275:1289-300.
44. Choi G, Song JS, Choi S-H, Nam SY, Kim SY, Roh J-L, et al. Comparison of Squamous Cell Carcinoma of the Tongue between Young and Old Patients. J Pathol Transl Med 2019; 53:369.
45. Marocchio LS, Giudice F, Corrêa L, Pinto Junior DdS, de Sousa SOM. Oestrogens and androgen receptors in oral squamous cell carcinoma. Acta Odontol Scand 2013; 71:1513-9.
46. Wu TF, Luo FJ, Chang YL, Huang CM, Chiu WJ, Weng CF, et al. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells. Oral Dis 2015; 21:320-7.
47. Nehse G, Tunn S. Androgen and progesterone receptors in oral carcinoma. J Craniomaxillofac Surg 1994; 22:114-9.
48. Ito FA, Ito K, Coletta RD, Vargas PA, Lopes MA. Immunohistochemical study of androgen, estrogen and progesterone receptors in salivary gland tumors. Braz Oral Res 2009; 23:393-8.
49. Aquino G, Collina F, Sabatino R, Cerrone M, Longo F, Ionna F, et al. Sex hormone receptors in benign and malignant salivary gland tumors: Prognostic and predictive role. Int J Mol Sci 2018; 19:399.
50. Moriki T, Ueta S, Takahashi T, Mitani M, Ichien M. Salivary duct carcinoma: cytologic characteristics and application of androgen receptor immunostaining for diagnosis. Cancer 2001; 93:344-50.
51. Fan C-Y, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol 2000; 24:579-86.
52. Fan C-Y, Melhem MF, Hosal AS, Grandis JR, Barnes EL. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor α in salivary duct carcinoma. Arch Otolaryngol Head Neck Surg 2001; 127:1075-9.
53. Sygut D, Bien S, Ziolkowska M, Sporny S. Immunohistochemical expression of androgen receptor in salivary gland cancers. Pol J Pathol 2008; 59:205-10.
54. Nakajima Y, Kishimoto T, Nagai Y, Yamada M, Iida Y, Okamoto Y, et al. Expressions of androgen receptor and its co-regulators in carcinoma ex pleomorphic adenoma of salivary gland. Pathology 2009; 41:634-9.
55. Godbole AM, Njar VC. New insights into the androgen-targeted therapies and epigenetic therapies in prostate cancer. Prostate Cancer 2011; 2011.
56. Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367:1187-97.
57. Group PCTC. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 2000; 355:1491-8.
58. Dos Santos M, Sibov T, Nishimoto I, Kowalski LP, Miracca E, Nagai
M. The CAG repeat polymorphism in the androgen receptor gene (AR) and its relationship to head and neck cancer. Oral Oncol 2004; 40:177-82.
59. Locati L, Quattrone P, Bossi P, Marchiano A, Cantu G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol 2003; 14:1327-8.